
If you had a rock that weighted 100g and contained 10g of gold, you would say that it was 10% ( w/w) ore. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. If you are making instant coffee, you might blend 10 g of Santa with 100 mLs of water. In that case you would have a 10% ( v/v) solution. You might mix 10 mLs of gin with 90 mLs of tonic. Solids are easier to weigh, while liquids are easier to measure volume. The theoretical (actual) percent hydration (percent water) can be calculated from the formula of the hydrate by dividing the mass of water in one mole of. These different variations of % are practical to chemists and cooks. There are a number of common methods for describing percent composition of solutions, such as: weight percent, volume percent, or weight/volume percent. ( v/v) Volume Solute / Volume Solution PercentĬhemists frequently like to express their concentration using terms of percentage.( w/w) Weight Solute / Weight Solution Percent.( w/v) Weight Solute / Volume Solution Percent.

The Measurement of Concentration Calculator computes the percent concentration based on an amount of solute and an amount of solution. Percent solute = x 100 = 10% calcium gluconate in solution mass of solution = (10g + 90g) = 100 grams.mass of solute = 10 grams calcium gluconate.So, if we wanted to use the percent by mass formula, it would look like this: In chemistry, unless stated otherwise, the solvent is assumed to be water. This is the same as 10 grams of calcium gluconate in 90 grams of water. ExampleĪ 10% calcium gluconate solution, Ca(C 6H 11O 7) 2, contains 10 grams by mass of calcium gluconate in 100 grams of solution. Each of these inputs have default units of grams, but can be calculated using any mass units (i.e., milligrams, kilograms.etc.). The mass of solution = mass of the solute + mass of solvent. The mass of solute should be directly given, however, the mass of solution can be calculated. percent composition ( 4 g / 345.25 g) x 100. percent composition (m solute / m solution) x 100. This method of expressing concentration is not only known as percent by mass, but also weight percent, volume percent, mass percent, and other variations of this. Step 4 - Determine percent composition by mass of the sugar solution. This formula gives the mass of solute per 100 units of solution.

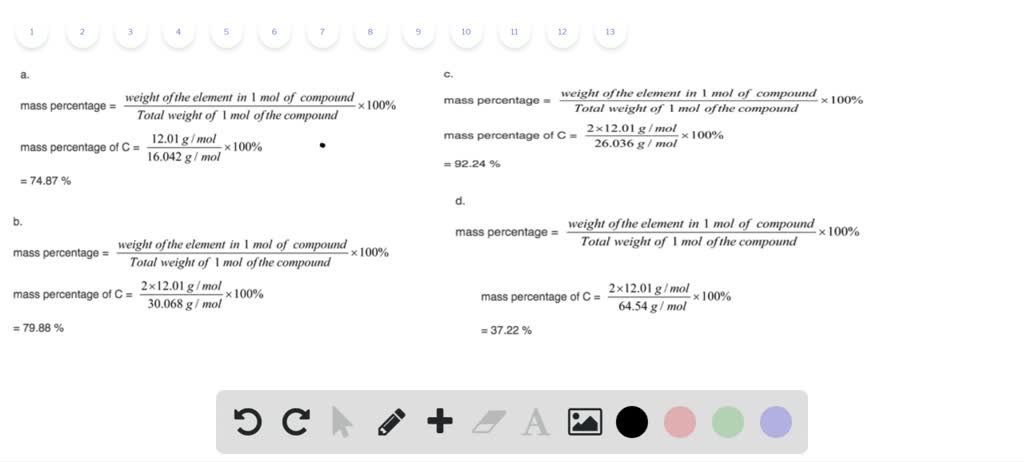
This is a method of expressing the concentration of a solution.


 0 kommentar(er)
0 kommentar(er)
